1.
Entamoeba histolyticaTransmission: Contaminated food/water, person to person, fecal exposure during sexual contact, rodents (vectors for transmitting
E. histolytica cysts)
Pathogenesis: Following ingestion, the cysts pass through the stomach, where exposure to gastric acid stimulates release of the pathogenic trophozoite in the duodenum. The trophozoites divide and produce extensive local necrosis in the large intestine. The basis of tissue destruction may be attributed to the production of a cytotoxin. Necrosis requires direct contact with the amoeba, so lysosomal enzymes (phospholipase A
2) may be important.
Epidemiology: Worldwide distribution. Its incidence is highest in tropical and subtropical regions that have poor sanitation and contaminated water.
Clinical syndromes: Intestinal or extraintestinal amebiasis (symptoms: abdominal pain, cramping, colitis with diarrhea, bloody stools, hepatomegaly)
Lab diagnosis: Identification of
E. histolytica trophozoites and cysts in stools and trophozoites in tissue under microscope, specific serologic tests, immunological tests for detection of fecal antigen and DNA-probe assays for
E. histolytica nucleic acids.
Treatment: Treated with metronidazole followed by iodoquinol
Prevention and control: Introduction of adequate sanitation measures and education about the routes of transmission, water should be boiled and fruits/vegetables should be thoroughly cleaned before consumption


2.
Giardia lambliaTransmission: Contaminated food/water, person to person, fecal-oral or oral-anal routes
Pathogenesis: Infection with
G. lamblia is initiated by ingestion of cysts. Gastric acid stimulates excystation with release of trophozoites in the duodenum and jejunum, where organisms multiply by binary fission. Trophozoites can attach to intestinal villi by a prominent ventral sucking disk.
Epidemiology: Worldwide distribution
Clinical syndromes: Result in either asymptomatic carriage or symptomatic disease, ranging from mild diarrhea to a severe malabsorption syndrome (symptoms: watery diarrhea, abdominal cramps, flatulence)
Lab diagnosis: Stool specimens should be examined for cysts and trophozoites under microscopy, specific serologic and immunological tests.
Treatment: Quinacrine, metronidazole
Prevention and control: Avoidance of contaminated food/water and high-risk sexual behaviour, public health efforts to identify reservoir of infection and proper functioning filtration systems in water supplies
Picture of
Giardia lamblia (
http://www.google.com/ > image >
Giardia lamblia)

3. Balantidium coli
Transmission: Swine and monkeys, fecal-oral route, contaminated water, person to person
Pathogenesis: Similar to amebiasis as organisms elaborate proteolytic and cytotoxic substances that mediate tissue invasion and intestinal ulceration
Epidemiology: Worldwide distribution
Clinical syndromes: Result in either asymptomatic carriage or symptomatic disease (symptoms: abdominal pain and tenderness, tenesmus, nausea, anorexia, watery stools with blood and pus, ulceration of intestinal mucosa)
Lab diagnosis: Microscopic examination of feces for trophozoites and cysts
Treatment: Tetracycline, iodoquinol and metronidazole
Prevention and control: Appropriate personal hygiene, maintenance of sanitary condtions and careful monitoring of pig feces
Picture of Balantidium coli (http://www.google.com/ > image > Balantidium coli)
4. Isospora belli
Transmission: Contaminated food/water, oral-anal sexual contact
Pathogenesis: Both sexual (gametogony) and asexual (schizogony) reproduction in the intestinal epithelium can occur. The end product of gametogenesis is the oocyst.
Epidemiology: Worldwide distribution
Clinical syndromes: May be asymptomatic carriers or gastrointestinal disease ranging from mild to severe. Disease most commonly mimics giardiasis, with a malabsorption syndrome characterized by loose, foul-smelling stools.
Lab diagnosis: Careful examination of concentrated stool sediment and special staining with either iodine or a modified acid-fast procedure will reveal the parasite.
Treatment: Trimethoprim sulfamethoxazole, combined pyrimethamine-sulfadiazine
Prevention and control: Maintain personal hygiene, high sanitary conditions and avoidance of oral-anal sexual contact
Picture of Isospora belli (http://www.google.com/ > image > Isospora belli)

5.
Cryptosporidium parvum
Transmission: Zoonotic spread from animal reservoirs to humans, person to person, fecal-oral and oral-anal routes
Pathogenesis: Typical of coccidians. In contrast to
Isospora,
Cryptosporidium is found within the brush border of the intestinal epithelium. The coccidian attach to the surface of the cells and replicate by a process that involves schizogony.
Epidemiology: Worldwide distribution
Clinical syndromes: May be asymptomatic carriers or mild self-limiting enterocolitis characterized by watery diarrhea without blood
Lab diagnosis: Stool examination, modified zinc sulfate centrifugal flotation technique or by Sheather’s sugar flotation procedure, specimens may be stained with modified acid-fast method or by an indirect immunofluorescence assay
Treatment: No broadly effective therapy
Prevention and control: Due to the widespread distribution of organism in humans and animals, preventing infection is difficult. Maintain personal hygiene, high sanitary conditions and avoidance of oral-anal sexual contact.
Picture of life cycle of
Cryptosporidium parvum (
http://www.google.com/ > image >
Cryptosporidium parvum)

6. Plasmodium species (P. vivax, P. falciparum, P. ovale, P. malariae)
Transmission: Via bite of female Anopheles mosquito vector
Pathogenesis: Infectious plasmodia sporozoites are introduced after a bite through the saliva of the Anopheles mosquito. The sporozoites are carried to the hepatocytes where asexual reproduction occurs. Some species (P. vivax and P. ovale) can establish a dormant hepatic phase in which hypnozoites do not divide. The hepatocytes eventually rupture, liberating merozoites which attach to specific RBC receptors and invade them, initiating the erythrocytic cycle. Asexual replication progresses through a series of stages (ring, trophozoite, schizont) that culminates in the rupture of the RBC, releasing more merozoites. Some merozoites also develop within RBCs into male and female gametocytes.
Epidemiology: Worldwide distribution depending on the type of species
Clinical syndromes: Fever, chills, rigors, muscle and joint pains, anaemia and convulsions. Severe malaria (usually caused by P. falciparum) may cause cerebral malaria, blackwater fever which could lead to coma, death.
Lab diagnosis: Thick and thin blood smears stained with Giemsa stain and observed under microscopy, rapid diagnostic kits
Treatment: Chloroquine, primaquine, mefloquine, quinine, doxycyline (however, there are increasing multidrug-resistant P. falciparum strains)
Prevention and control: Chemoprophylaxis and prompt eradication of infections, control of mosquito breeding and protection of individuals by screening, netting, protective clothing and insect repellents.
Picture of life cycle of Plasmodium species (http://www.google.com/ > image > Plasmodium)

7. Toxoplasma gondii
Transmission: Ingestion of improperly cooked meat from animals serving as intermediate hosts or infective oocysts from cat fecal contamination, transplacental infection
Pathogenesis: Organisms develop in the intestinal cells of the cat, as well as during an extraintestinal cycle with passage to the tissues via bloodstream. The organisms from the intestinal cycle are passed in cat feces and mature in the external environment within 3 to 4 days into infective oocysts. These oocysts can be ingested by mice and other animals (including huamsn) and produce acute and chronic infection of various tissues.
Clinical syndromes: May be asymptomatic carriers or symptomatic disease (symptoms: chills, fever, headaches, myalgia, lymphadenitis)
Lab diagnosis: Serologic tests, ELISA test for detecting IgM antibodies, biopsy specimens
Treatment: Depends on the nature of both the infectious process and the immunocompetence of the host
Prevention and control: Avoidance of consumption and handling of raw or undercooked meat and exposure to cat feces
Picture of Toxoplasma gondii (http://www.google.com/ > image > Toxoplasma gondii)

8. Leishmania species
Transmission: Via sandflies (Phlebotomus or Lutzomyia)
Pathogenesis: The hemoflagellates are flagellated insect-transmitted protozoa that infect blood and tissues. The diseases are distinguished by the ability of the organism to infect deep tissues (visceral leishmaniasis) or replicate only in cooler superficial tissues (cutaneous or mucocutaneous leishmaniasis). The reservoir hosts and geographical distribution differ for the 3 species. There are two morphological forms in the cells: promastigote (with an anterior flagellum) in the insect host, and amastigote (without flagella) in the vertebrate host.
Clinical syndromes: Visceral (fever, swelling of spleen and liver, anaemia), cutaneous/mucocutaneous (skin reactions)
Lab diagnosis: Amastigote stafe can be demonstrated in tissue biopsy, bone marrow examination, lymph node aspiration and through examination of properly strained smears. Culture of blood, bone marrow and other tissues often demonstrates the promastigote stage. Specific serologic testing is also available.
Treatment: Stibogluconate
Prevention and control: Protection from sandflies by screening and use of insect repellents
Picture of Leishmania species (http://www.google.com/ > image > Leishmania)

9. Wuchereria bancrofti and Brugia malayi
Transmission: Via bite of Anopheles, Aedes and Culex mosquitoes
Pathogenesis: The infective larvae migrate from the location of bite to the lymphatics, where growth to adulthood occurs. For 3 to 12 months after the initial infection, the adult male worm fertilizes the female, who in turn produces the sheathed larval microfilariae that find their way into the circulation. Both species have a periodicity in production of microfilariae (nocturnal periodicity) which results in greater numbers of microfilariae in blood at night.
Epidemiology: W. bancrofti occurs in both tropical and subtropical areas and is endemic in central Africa and many parts of Asia with no animal reservoir identified. B. malayi is found primarily in parts of Asia, while cats and monkeys are recognized as animal reservoirs.
Clinical syndromes: Fever, lymphanigitis, filarial elephantiasis of extremities
Lab diagnosis: Eosinophilia present, thick and thin blood smears stained with Giemsa stained under microscopy, anticoagulated blood and urine specimens, specific serological testing
Blood specimens are recommended to be taken between 10pm – 4am.
Treatment: Diethylcarbamazine
Prevention and control: Education regarding filarial infections, mosquito control, use of protective clothing and insect repellents, treatment of infections to prevent further transmission
Picture of microfilariae (http://www.google.com/ > image > microfilariae)

References:
- Murray, P.R., Kobayashi, G.S., Pfaller, M.A., Rosenthal, K.S. (1994). Medical Microbiology Second Edition. London: Mosby-Year Book, Inc
- Medical Microbiology Notes
- http://www.wikipedia.org/ > search
Researched by: Alex Tan 0503222B













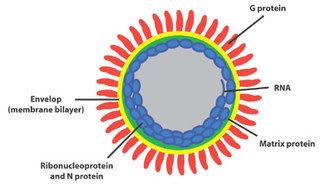
 A microscopic image shows poliomyelitis viruses, which enter the body through the nose and mouth and destroy nerve cells by multiplying rapidly inside of them. It can cause permanent paralysis. An effective vaccine have developed and poliomyelitis has been nearly eliminated in developed countries.
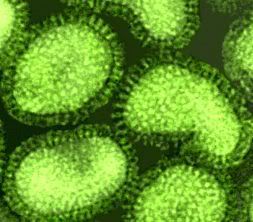
A microscopic image shows poliomyelitis viruses, which enter the body through the nose and mouth and destroy nerve cells by multiplying rapidly inside of them. It can cause permanent paralysis. An effective vaccine have developed and poliomyelitis has been nearly eliminated in developed countries. Hepatitis A magnified 225,000 times
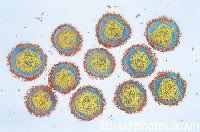
Hepatitis A magnified 225,000 times Hepatitis E
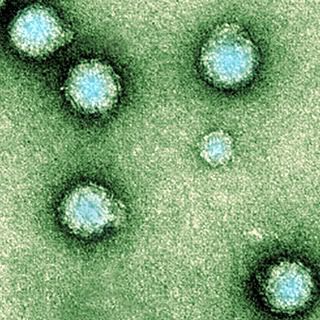
Hepatitis E An electron micrograph of Yellow Fever Virus virions. Virions are spheroidal, uniform in shape and are 40-60nm in diameter. The name "Yellow Fever" is due to the ensuing jaundice that affects some patients. The vector is the Aedes aegypti or Haemagogus spp. mosquito
An electron micrograph of Yellow Fever Virus virions. Virions are spheroidal, uniform in shape and are 40-60nm in diameter. The name "Yellow Fever" is due to the ensuing jaundice that affects some patients. The vector is the Aedes aegypti or Haemagogus spp. mosquito Schematic illustration of the relation between climate and the transmission of tick-borne diseases in humans. In this study, the different direct and indirect climate-dependent interactions, here shown within the yellow field, have been treated as a black box.
Schematic illustration of the relation between climate and the transmission of tick-borne diseases in humans. In this study, the different direct and indirect climate-dependent interactions, here shown within the yellow field, have been treated as a black box.